[ad_1]

The investigators identified new biological markers that may predict which ovarian cancer patients won’t respond to chemotherapy. Chowdhury et al, Cell.
NEW YORK (PRWEB)
August 03, 2023
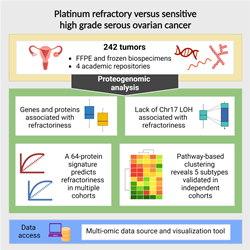
Using a novel proteogenomic strategy and a variety of machine learning tools, investigators from the Icahn School of Medicine at Mount Sinai and colleagues have identified a 64-protein signature that may predict a subset of ovarian cancer patients who are unlikely to respond to chemotherapy.
The multicenter study, published online August 3 in Cell [DOI#: 10.1016/j.cell.2023.07.004], reports on a pioneering analysis of chemo-refractoriness in high-grade serous ovarian cancer (HGSOC). The work also implicates possible therapeutic targets for these patients.
Epithelial ovarian cancer causes 185,000+ global deaths annually. HGSOC accounts for 60 percent of these deaths. Despite advances in treatment, mortality has remained the same for these patients in the past 40 years. Currently, there’s no way to distinguish refractory cases (who never respond to chemotherapy), leading some patients to unnecessarily experience the adverse effects of platinum-based chemotherapy without the benefits.
“To address this critical unmet need, we performed a proteogenomic analysis to identify molecular signatures of refractory HGSOC and potential treatment targets. Predictors of chemo-refractoriness could enable precision oncology, sparing patients the toxicity and helping to identify the most effective therapy through targeted clinical trials,” says Pei Wang, PhD, Professor of Genetics and Genomic Sciences at Icahn Mount Sinai and co-corresponding author on the paper.
The investigators studied 242 tumors samples collected from HGSOC patients comprising both chemo-refractory and chemo-responsive individuals before they received chemotherapy. Using advanced computer models to analyze protein and gene expression profiles of the tumors, they found a specific group of 64 proteins that can predict which tumors won’t respond well to the first-line platinum-based therapy. This prediction was confirmed in two independent cohorts of patients.
In addition, based on pathway activity measurements derived from the proteomics data, the team also identified five new HGSOC subtypes, validated in two independent patient groups and in lab-grown tumor mouse models, suggesting that different treatment strategies may be needed.
Next, the researchers plan to confirm their findings in additional retrospective and prospective studies.
Once validated, these tools, say the investigators, can be used by clinicians to design customized alternative treatments other than the current standard chemotherapy to help patients with refractory tumors.
As a part of the research, the lab of Amanda Paulovich, MD, PhD, a lead author of the study, is working on a new test that uses a multiplex assay panel to measure the proteins in the prediction model faster and more efficiently. Dr. Paulovich is a professor at Fred Hutchinson Cancer Center in Seattle, where she holds the Aven Foundation Endowed Chair.
The test will combine information from multiple proteins to create a single score that indicates the likelihood of chemo-refractory disease. If successful, say the investigators, it could be a significant development for about 35 percent of patients with ovarian cancer who could avoid treatments that won’t work for their specific type of cancer.
The paper is titled “Proteogenomic analysis of chemo-refractory high-grade serous ovarian cancer.”
Authors on the Cell paper include: Shrabanti Chowdhury, PhD, (Icahn Mount Sinai); Jacob J. Kennedy (Fred Hutchinson Cancer Center); Richard G. Ivey (Fred Hutchinson Cancer Center); Oscar D. Murillo (Fred Hutchinson Cancer Center); Noshad Hosseini, PhD candidate (University of Michigan School of Medicine); Xiaoyu Song, DrPH (Icahn Mount Sinai); Francesca Petralia, PhD (Icahn Mount Sinai); Anna Calinawan (Icahn Mount Sinai); Sara R. Savage, PhD (Baylor College of Medicine); Anna B. Berry, MD (Syapse, Inc.); Boris Reva, PhD (Icahn Mount Sinai); Umut Ozbek, PhD (Icahn Mount Sinai); Azra Krek, PhD (Icahn Mount Sinai); Weiping Ma, PhD (Icahn Mount Sinai); Felipe da Veiga Leprevost, PhD (University of Michigan School of Medicine); Jiayi Ji (Icahn Mount Sinai); Seungyeul Yoo, PhD (Sema4); Chenwei Lin, PhD (Fred Hutchinson Cancer Center); Uliana J. Voytovich (Fred Hutchinson Cancer Center); Yajue Huang, MD (Mayo Clinic); Sun-Hee Lee, PhD (Mayo Clinic); Lindsay Bergan (Fred Hutchinson Cancer Center); Travis D. Lorentzen (Fred Hutchinson Cancer Center); Mehdi Mesri, M.Med.Sci.PhD (National Cancer Institute); Henry Rodriguez, PhD, MS, MBA (National Cancer Institute); Andrew N. Hoofnagle, MD, PhD (University of Washington); Zachary T. Herbert, MS (Dana-Farber Cancer Institute); Alexey I. Nesvizhskii, PhD (University of Michigan School of Medicine); Bing Zhang, PhD (Baylor College of Medicine); Jeffrey R. Whiteaker, PhD (Fred Hutchinson Cancer Center); Samuel C. Mok, PhD (The University of Texas MD Anderson Cancer Center); Scott H. Kaufmann, MD, PhD (Mayo Clinic); Charles Drescher, MD (Fred Hutchinson Cancer Center); Marcin Cieslik, PhD (University of Michigan School of Medicine); Pei Wang, PhD (Icahn Mount Sinai); Michael J. Birrer, MD, PhD (University of Arkansas for Medical Sciences); Amanda G. Paulovich, Md, PhD (Fred Hutchinson Cancer Center); and Clinical Proteomic Tumor Analysis Consortium (CPTAC) Collaborators (NYU School of Medicine, University of Miami, University of California San Francisco, and University of Minnesota).
This work was done in collaboration with the U.S. National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC) and supported by grants U01CA214114, R50CA211499, U24CA210993, U24CA271114, U24CA210954, U24CA210967, U24CA210972, P30CA240139, P50CA136393, S10OD028685, and P30CA015704, as well as from a generous donation from the Aven Foundation.
To view competing interests, please see the paper at Cell.
About the Icahn School of Medicine at Mount Sinai
The Icahn School of Medicine at Mount Sinai is internationally renowned for its outstanding research, educational, and clinical care programs. It is the sole academic partner for the eight- member hospitals* of the Mount Sinai Health System, one of the largest academic health systems in the United States, providing care to a large and diverse patient population.
Ranked 14th nationwide in National Institutes of Health (NIH) funding and among the 99th percentile in research dollars per investigator according to the Association of American Medical Colleges, Icahn Mount Sinai has a talented, productive, and successful faculty. More than 3,000 full-time scientists, educators, and clinicians work within and across 44 academic departments and 36 multidisciplinary institutes, a structure that facilitates tremendous collaboration and synergy. Our emphasis on translational research and therapeutics is evident in such diverse areas as genomics/big data, virology, neuroscience, cardiology, geriatrics, as well as gastrointestinal and liver diseases.
Icahn Mount Sinai offers highly competitive MD, PhD, and Master’s degree programs, with current enrollment of approximately 1,300 students. It has the largest graduate medical education program in the country, with more than 2,000 clinical residents and fellows training throughout the Health System. In addition, more than 550 postdoctoral research fellows are in training within the Health System.
A culture of innovation and discovery permeates every Icahn Mount Sinai program. Mount Sinai’s technology transfer office, one of the largest in the country, partners with faculty and trainees to pursue optimal commercialization of intellectual property to ensure that Mount Sinai discoveries and innovations translate into healthcare products and services that benefit the public.
Icahn Mount Sinai’s commitment to breakthrough science and clinical care is enhanced by academic affiliations that supplement and complement the School’s programs.
Through the Mount Sinai Innovation Partners (MSIP), the Health System facilitates the real-world application and commercialization of medical breakthroughs made at Mount Sinai. Additionally, MSIP develops research partnerships with industry leaders such as Merck & Co., AstraZeneca, Novo Nordisk, and others.
The Icahn School of Medicine at Mount Sinai is located in New York City on the border between the Upper East Side and East Harlem, and classroom teaching takes place on a campus facing Central Park. Icahn Mount Sinai’s location offers many opportunities to interact with and care for diverse communities. Learning extends well beyond the borders of our physical campus, to the eight hospitals of the Mount Sinai Health System, our academic affiliates, and globally.
——————————————————-
- Mount Sinai Health System member hospitals: The Mount Sinai Hospital; Mount Sinai Beth Israel; Mount Sinai Brooklyn; Mount Sinai Morningside; Mount Sinai Queens; Mount Sinai South Nassau; Mount Sinai West; and New York Eye and Ear Infirmary of Mount Sinai.
[ad_2]