[ad_1]

G-CON Standard PODs
The Standard POD launch has been a major goal for us, and allows us to mass produce, which will reduce the delivery timeline and cost of cleanrooms.
COLLEGE STATION, Texas (PRWEB)
September 08, 2020
G-CON Manufacturing, the global leader in prefabricated, flexible cleanroom solutions, today unveiled its new Standard POD product line. The Standard POD designs will maximize efficiencies related to production, qualification, delivery and cost reduction and eliminate the complexity of cleanroom construction projects.
G-CON’s standard POD portfolio encompasses six PODs, three different dimensions with bi-directional and uni-directional flow approaches and all featuring ISO 7 classification. These units can ably support development and clinical scale operations for cell therapies, formulation and filling, drug substance, etc. and meet the building block approach to allow for configuration and assembly of multiple units to meet clients’ segregation and space requirements.
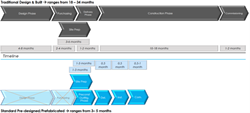
PODs, which have gained accelerating popularity over the past 10 years due to their prefabricated and prequalified turnkey benefits, are now available as an off-the-shelf piece of equipment through the Standard POD product line. With standardization, these cleanrooms can be purchased at a set list price, inclusive of design and qualification, much like purchasing a car. In this approach, lengthy design phases are unnecessary, and the product is produced repetitively with standard parts from qualified vendors by an experienced work force. Commissioning and qualification are routine as well resulting in a delivery of three months from order.
“At G-CON we constantly strive to deliver biopharmaceutical processing spaces faster, as time is of essence for our clients and patients,” Maik Jornitz, CEO of G-CON stated. “The Standard POD launch has been a major goal for us, and allows us to mass produce, which will reduce the delivery timeline and cost of cleanrooms. These PODs will also provide repeatable CGMP compliant quality, instead of the variable results that occur with other cleanroom approaches.”
The launch advances G-CON’s recently announced BUILDING FOR LIFE™ campaign, a campaign dedicated to getting drug therapies to patients faster.
To request more information or to receive our Standard POD Catalog, please email sales@gconbio.com.
About G-CON Manufacturing
G-CON Manufacturing designs, builds and installs prefabricated G-CON POD® cleanrooms. G-CON’s POD portfolio provides cleanrooms in a number of dimensions for a variety of uses, from laboratory environments to personalized medicine and production process platforms. G-CON POD® cleanroom units surpass traditional cleanroom structures in scalability, mobility and the possibility of repurposing the PODs once the production process reaches its lifecycle end. For more information, please visit G-CON’s website at http://www.gconbio.com.
G-CON Manufacturing… BUILDING FOR LIFE
Share article on social media or email:
[ad_2]