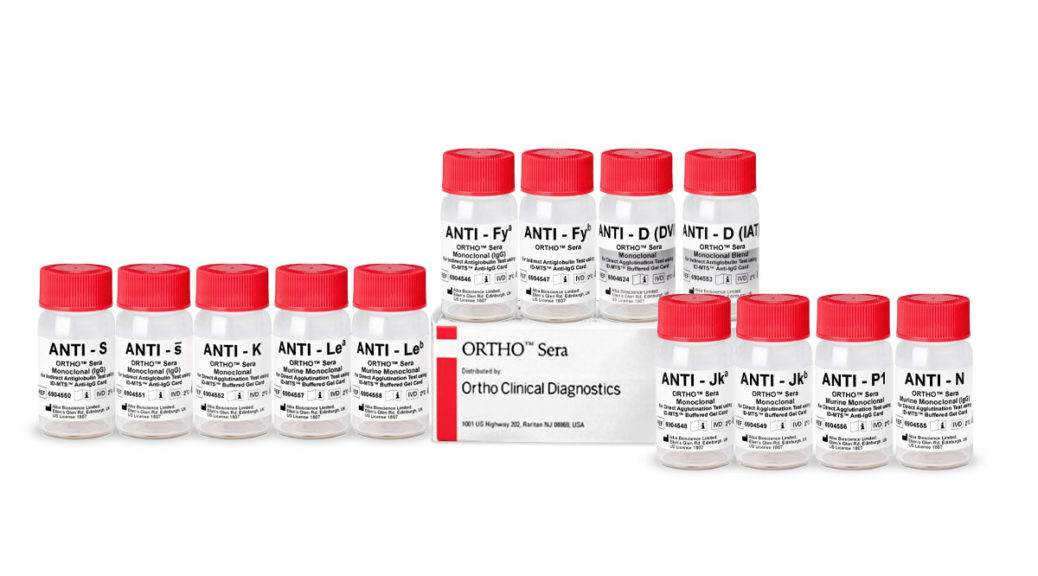
Ortho Sera
Ortho Clinical Diagnostics is proud to offer our laboratory partners solutions that help them quickly test donor blood for the most-needed phenotypes and confidently provide it to patients in need of transfusion.
RARITAN, N.J. (PRWEB)
March 10, 2020
Ortho Clinical Diagnostics, a global leader of in vitro diagnostics dedicated to improving and saving lives through innovative laboratory solutions, announced its ORTHOTM Sera, ID-MTS, a suite of extended antigen in a gel testing format used for blood phenotyping for patients in need of transfusion, is cleared to run on ORTHO VISION® and ORTHO VISION® Max in the United States and Canada. The clearances allow testing for more than 99 percent of the most commonly tested blood group antigens to be performed on one platform, driving greater value and efficiency.
The suite was cleared by the U.S. Food and Drug Administration for use on the ORTHO VISION® Analyzer in October 2019, and for use on the ORTHO VISION® Max in January 2020. The suite received Health Canada approval in January 2020.
Watch a video on how Sera works: https://www.youtube.com/watch?v=wL2oVvFXhsY.
“Ortho Clinical Diagnostics is proud to offer our laboratory partners solutions that help them quickly test donor blood for the most-needed phenotypes and confidently provide it to patients in need of transfusion,” said Bob Stowers, head of Ortho’s transfusion medicine product portfolio. “With the ability to analyze the most commonly tested antigens on a single ORTHO VISION system and results in under 25 minutes, lab teams are better equipped to help clinicians focus on what matters most—treating their patients.”
During pre-transfusion testing, lab professionals routinely encounter complex patient samples that require extended antigen typing. These patients have developed atypical antibodies to blood group antigens and require additional testing to find compatible blood.
Its antisera additive approach means customers always have the appropriate reagents available and allows extended phenotyping to be personalized according to patient needs—reducing waste and enhancing efficiency.
With end-to-end full automation on the ORTHO VISION and ORTHO VISION Max analyzers, ORTHO Sera minimizes the potential for human error—improving safety—and provides consistent, reliable results while freeing up lab professionals to focus on value-added tasks.
The 13 ORTHO Sera reagents available in the U.S. and Canada on the ORTHO VISION Analyzer and ORTHO VISION Max include Anti-D (DVI), Anti-D (IAT), Anti-Fya, Anti-Fyb, Anti-Jka, Anti-Jkb, Anti-K, Anti-Lea, Anti-Leb, Anti-N, Anti-P1, Anti-S, Anti-s.
For more information, visit https://www.orthoclinicaldiagnostics.com/en-us/home/ortho-sera.
About Ortho Clinical Diagnostics
Ortho Clinical Diagnostics, a global leader of in vitro diagnostics dedicated to improving and saving lives through innovative laboratory solutions, serves the clinical laboratory and immunohematology communities.
Ortho’s high-quality products and services help hospitals, hospital networks, blood banks and labs in more than 125 countries and territories deliver consistently fast, accurate, and reliable results that allow clinicians to make better-informed treatment decisions.
Ortho’s lab solutions provide sophisticated testing technologies, automation, information management and interpretation tools to clinical laboratories to help them run more efficiently and effectively and improve patient care.
Ortho’s blood typing products help ensure every patient receives blood that is safe, the right type and the right unit.
Why does Ortho continue to innovate and provide industry-changing solutions? Because every test is a life.
For more information about Ortho’s solutions and services, visit http://www.orthoclinicaldiagnostics.com, or visit Ortho on social media: LinkedIn, Twitter, Facebook, Instagram and YouTube.
Share article on social media or email: