[ad_1]

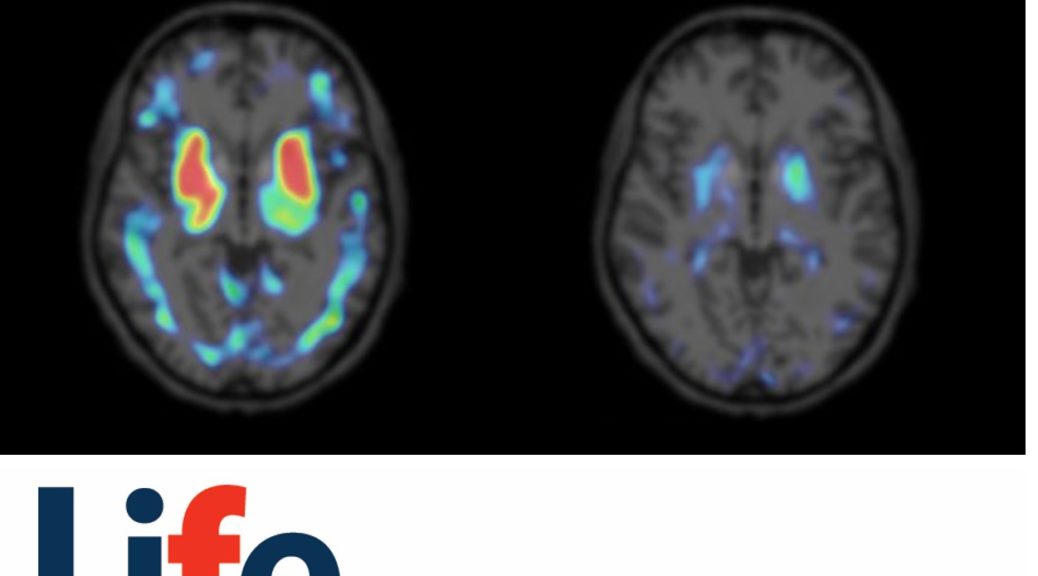
PI-2620 TAU-PET
It is a big step forward to finally have an objective diagnostic procedure within reach for assessment of tau pathology in various cortical and subcortical sites.
BERLIN (PRWEB)
May 20, 2021
Life Molecular Imaging (LMI) announces today that PI-2620 has been granted four different orphan drug designations, two by the European Commission and two by the U.S. Food and Drug Administration. The designations in Europe cover diagnosis of Progressive Supranuclear Palsy (PSP) and diagnosis of Corticobasal Degeneration (CBD). The U.S. designations have been granted for the clinical management of PSP and clinical management of CBD. With this, PI-2620 is the first and only imaging agent for positron emission tomography (PET) to receive orphan drug designation for the diagnosis of PSP and CBD.
PSP and CBD are both rare and rapidly progressive neurodegenerative diseases that can lead to premature death. It can be challenging to diagnose patients based on symptoms, such as movement difficulties, stiffness, clumsiness, cognitive impairment and frequent falls, because they are also present in other, non-tau-related neurodegenerative diseases, like Parkinson’s disease (PD). Misdiagnosis of PSP and CBD, especially in the early stages of the diseases, hampers drug development due to heterogeneous study populations that inadvertently mix patients with tau-related pathologies with patients suffering from other proteinopathies.
The orphan drug designations are accompanied by recent clinical data, which established the ability of PI-2620 to reliably detect the characteristic 4-repeat (4R)-tau depositions in PSP and CBD cohorts through PET imaging [1,2]. These seminal clinical results further demonstrate PI-2620’s excellent characteristics as a unique diagnostic tool for studying 4R-tau-related diseases, in addition to its strong performance in AD [3]. PI-2620 can aid in the diagnosis of PSP and CBD patients earlier and more reliably, allowing differentiation of these tauopathies from other neurodegenerative diseases [1,2].
“The orphan medicinal product designations for PI-2620 strengthen our development program for the indications of PSP and CBD. This innovative and differentiated diagnostic tool is available to physicians and patients worldwide and can already be used as investigational medicinal product in research studies with tauopathy patients. The latest clinical results encourage further research and development of this imaging technology towards a fully validated tracer. Once approved, PI-2620 may facilitate earlier and more accurate diagnosis of not only Alzheimer’s Disease, but also PSP and CBD, enabling better management of these diseases,” said Andrew Stephens, MD, PhD, Chief Medical Officer of LMI.
Prof. Dr. Günter Höglinger, Director of the Department of Neurology at Hannover Medical School, added: “It is a big step forward to finally have an objective diagnostic procedure within reach for assessment of tau pathology in various cortical and subcortical sites. The next important steps are to investigate the usefulness of PI-2620 as a progression biomarker and as a possible target-engagement marker for therapeutic studies.”
References
1. Brendel M et al. “Assessment of 18F-PI-2620 as a Biomarker in Progressive Supranuclear Palsy” JAMA Neurology 77(11):1408-1419 https://doi.org/10.1001/jamaneurol.2020.2526.
2. Palleis C, et al. “Cortical [18 F]PI-2620 Binding Differentiates Corticobasal Syndrome Subtypes” Mov Disord. 2021 May 5. https://doi.org/10.1002/mds.28624.
3. Mueller A et al. “Tau PET imaging with 18F-PI-2620 in Patients with Alzheimer Disease and Healthy Controls: A First-in-Humans Study” J Nucl Med. 2020 Jun;61(6):911-919. https://jnm.snmjournals.org/content/61/6/911.
About Life Molecular Imaging (LMI)
Life Molecular Imaging (LMI, formerly Piramal Imaging) was formed in 2012 with the acquisition of the molecular imaging research and development portfolio of Bayer Pharma AG. It is now part of the Alliance Medical Group (a member of the Life Healthcare Group) offering an integrated business including research and development laboratories, a network of cyclotrons, radiopharmacies and imaging facilities. By developing novel PET tracers for molecular imaging, LMI is focusing on a key field of modern medicine. The organization strives to be a leader in the Molecular Imaging field by developing innovative products that improve early detection and characterization of chronic and life-threatening diseases, leading to better therapeutic outcomes and improved quality of life.
Please visit https://life-mi.com.
About Life Healthcare Group
Life Healthcare Group is a market-leading, international, diversified healthcare organization. Life Healthcare has over 33 years’ experience in the South African private healthcare sector, and currently operates 66 healthcare facilities in southern Africa. Services include acute hospital care, acute physical rehabilitation, acute mental healthcare, renal dialysis, and employee health and wellness services. The Group owns Alliance Medical Group, the leading independent provider of medical imaging services within Europe, operating across 10 international countries. Visit http://www.lifehealthcare.co.za
About PI-2620
PI-2620 was discovered in a research collaboration with Life Molecular Imaging and AC Immune. Life Molecular Imaging has the exclusive world-wide license for research, development and commercialization of tau-PET tracers generated within the discovery program. PI-2620 is currently under investigation in several clinical studies as a targeted radiopharmaceutical for the detection of tau deposits in the human brain.
Media Contact:
Nicole Fletcher | Marketing Communications | Life Molecular Imaging
+1 857-202-1122 | n.fletcher@life-mi.com
Share article on social media or email:
[ad_2]