[ad_1]

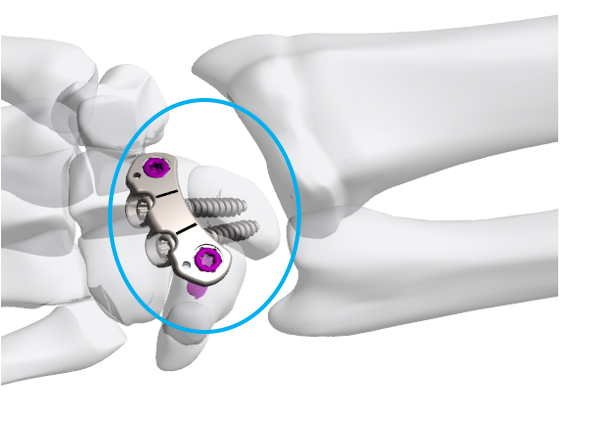
ExsoMed’s Four Corner Fusion System
“ExsoMed’s Four Corner Fusion System brings an innovative problem-specific solution that offers the patient and the surgeon benefits beyond anything else in the market,” said Martin I. Boyer, MD, FRCS(C) of Washington University School of Medicine in Saint Louis, MO.
ALISO VIEJO, Calif. (PRWEB)
September 09, 2020
ExsoMed Corporation, a privately held U.S.-based medical device company providing orthopaedic surgeons with innovative solutions in hand surgery, received 510(k) clearance from the United States Food and Drug Administration for its Four Corner Fusion System to treat wrist arthritis.
Four Corner Fusion has shown to be a reliable option to treat wrist arthritis, however current product offerings still have many shortcomings, without much innovation being developed to address these issues. ExsoMed’s Four Corner Fusion System will provide surgeons with a solution that is simple to use, that requires less reaming, less “drill and fill”, no impingement on the lunate, and preserves the cartilage.
“I am pleased with the result of our work with ExsoMed,” said Martin I. Boyer, MD, FRCS(C) of Washington University School of Medicine in Saint Louis, MO. “This new Four Corner Fusion System brings an innovative problem-specific solution that offers the patient and the surgeon benefits beyond anything else in the market. Our system specifically preserves the articular cartilage of the proximal aspect of the lunate and corrects the capitolunate malalignment in both coronal and sagittal planes.”
In addition to Dr. Boyer, Kavi Sachar, MD of the Steadman Clinic in Vail, CO and Kathleen McKeon, MD of the Andrews Sports Medicine and Orthopaedic Center in Birmingham, AL were instrumental in the design of this product.
“ExsoMed is thrilled to have partnered with some of hand surgery’s brightest minds to develop this innovative Four Corner Fusion plating system,” said William E. Maya, ExsoMed’s Chief Executive Officer. “We believe the product brings significant advancement to what is often a difficult procedure with poor outcomes. The system is through the FDA clearance and patent processes; however, we have made a strategic business decision to stay focused on our market-differentiating, sterile-packed, single-use business model. ExsoMed will begin strategic discussions to sell this asset and ensure that it will be made available to enhance outcomes for surgeons and patients.”
For more information on ExsoMed’s Four Corner Fusion System, please contact James Kim at jkim@exsomed.com.
About ExsoMed
ExsoMed is a privately held medical device company providing orthopaedic surgeons with innovative solutions in hand surgery. We believe that our solutions raise the standard of care in hand surgery by providing state of the art surgical tools that streamline use in the operating room, reduce the global cost of care, and enhance outcomes so that patients can get back to life faster. For more information regarding ExsoMed, please visit http://www.exsomed.com.
“ExsoMed™ is a trademark of ExsoMed Corporation”
Share article on social media or email:
[ad_2]