Airs on Lifetime TV at 7:30am EST
“To be a patient advocate is to arm yourself with the latest research, knowledge and resources and so far we’ve been able to touch the lives of millions of people through the 85 disease specific segments we’ve aired so far,” says Carri Levy, Creator – Behind The Mystery: Rare and Genetic Series.
DEERFIELD BEACH, Fla. (PRWEB)
September 18, 2019
Did you know that one in 10 Americans are living with a rare disease? Rare and genetic diseases often don’t get the attention they deserve and can have devastating effects on patients, their families and caregivers. That’s why for the past eight years, The Balancing Act’s series, Behind The Mystery: Rare and Genetic, has been bringing these often undiagnosed, misdiagnosed and complex medical conditions to the forefront by partnering with pharmaceutical and biotechnology companies who are on a mission to educate the public, which should lead to earlier diagnosis, treatment and provide a community for people suffering from these disorders.
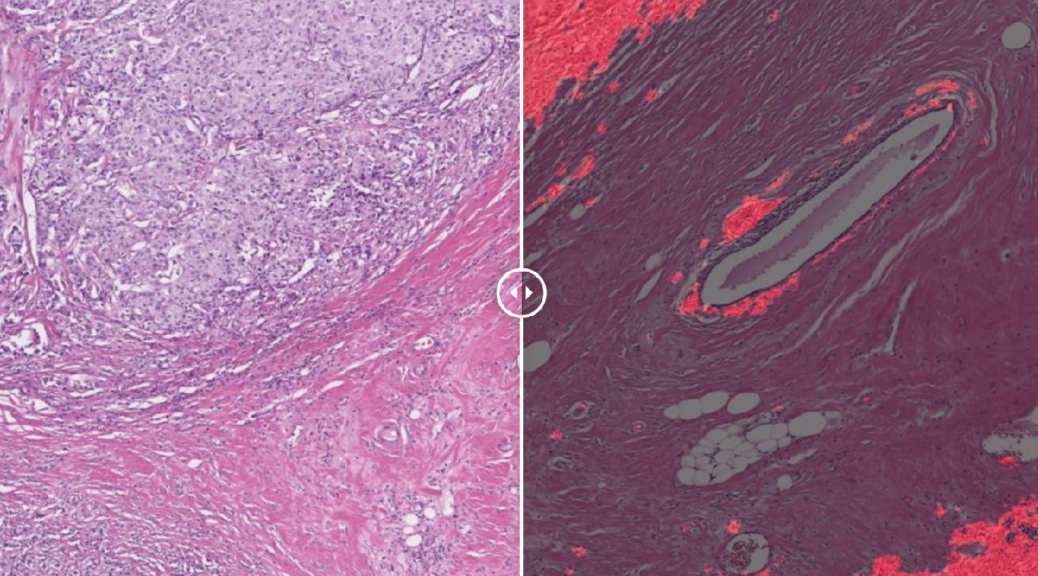
The upcoming extended 30 minute “Special Edition” of Behind The Mystery: Rare and Genetic will examine ADPKD – Autosomal Dominant Polycystic Kidney Disease – a rare genetic disease that can lead to end state renal disease and BPDCN – Blastic Plasmacytoid Dendritic Cell Neoplasm, an aggressive blood cancer that is often times fatal if left untreated,. The special will witness real life conversations with experts at medical research institutions. Viewers will hear from patients who are living with these conditions and learn from their experiences. The show’s website (thebalancingact.com/rare) is a valuable 24/7 resource for patients and doctors who want to learn more about the over 85+ featured rare and genetic diseases.
The creator of the show, Carri Levy, is actively involved in the rare disease community because she knows first-hand how important it is to find answers for rare medical conditions, through her journey with her own daughter. Levy created Behind The Mystery: Rare and Genetic with her co-producer, Molly Mager, whose twin brother has an ultra-orphan disease, to help other families, like her own, navigate the difficult path toward diagnosis of one of the 7,000 rare and genetic diseases known.
The “Special Edition” Behind The Mystery: Rare and Genetic will air on Tuesday, September 24th on Lifetime at 7:30am EST. Previously aired episodes may be viewed at TheBalancingAct.com.
About The Balancing Act: The Balancing Act is a daily morning show created and produced by BrandStar that brings fresh ideas to today’s modern woman to help balance and enrich her life every day. Now in its ninth season, The Balancing Act features everything from delicious recipes, style makeovers and dream getaways to parenting tips and the latest news in health and wealth. Tune in to The Balancing Act, America’s premier half-hour show for women and about women, weekday mornings at 7:30 a.m. (ET/PT) on Lifetime®.
Share article on social media or email: