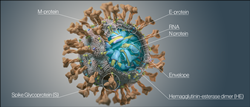
SARS-CoV-2 Antigens
Quantitative antibody tests are the primary tool clinicians and researchers use to measure our immune response to both infections and vaccines.
OAKLAND, Calif. and WENDELSHEIM, Germany (PRWEB)
July 09, 2020
Shortly after infection with SARS-CoV-2, the virus that causes COVID-19, our immune system reacts with IgM and/or IgA antibodies. Once the virus is detected and attacked, the body produces specific IgG to eliminate the virus and maintain some level of protection against future exposure to SARS-CoV-2. Vaccines similarly work by teaching the immune system to make antibodies to protect against specific pathogens. Quantitative antibody tests are the primary tool clinicians and researchers use to measure our immune response to both infections and vaccines.
Antibody measurement requires carefully sifting through the many potential targets and selecting those most immunogenic and unique for the pathogen of interest. Immunogenicity is important because higher levels of antibodies help improve differentiation between positive and negative samples, improving sensitivity. Finding specific proteins reduces the risk of cross-reaction with antibodies to other pathogens, improving specificity. For a SARS-CoV-2 antibody test, the two most established targets are the nucleocapsid protein (NP) and the spike protein (S1).
NP has been demonstrated to be the most sensitive antibody target so NP antibody tests might support earlier identification, but the protein is highly conserved in the coronavirus family, increasing the risk of cross-reaction with non-specific antibodies. Anti-NP antibodies have also not been demonstrated to have a neutralizing effect, so their measurement may not be appropriate to help assess immunity.
S1 has been demonstrated to be highly specific for the SARS-CoV-2 virus lowering the risk of cross-reactions with antibodies against other members of the coronavirus family. S1 contains the receptor-binding domain (RBD) so antibodies against it may be neutralizing.
Additional information about SARS-CoV-2 antibody testing with NP and S1 proteins can be found here.
AESKU.Diagnostics will offer six different SARS-CoV-2 antibody assays, AESKULISA® SARS-CoV-2 NP IgM/ IgA / IgG and AESKULISA® SARS-CoV-2 S1 IgM/ IgA / IgG. IgG and IgM SARS-CoV-2 antibody assays qualify for EUA FDA submission, but IgA assays currently do not.
At a glance:
Advantages of the AESKULISA® SARS-CoV-2 immunoassays at a glance:
- First quantitative test in the market
- Room Temperature incubation
- Easy to automate, optimized for use with any ELISA platform
- Breakaway wells allow customizable panels
- Assay Protocol Files available
- Common reagents
- Optimized incubation (30 min, 30 min, 30 min)
- Nucleocapsid Protein IgA / IgG/ IgM
or
- Spike-Protein S1 IgA / IgG/ IgM
Submission number:EUA201942
ABOUT AESKU
AESKU.GROUP is headquartered in Wendelsheim, Germany and is made up of the following divisions: AESKU.DIAGNOSTICS, AESKU.THERAPY (Autoimmune Therapies), AESKU.SYSTEMS (Lab Automation), AESKU.INC (N. American headquarters in Oakland, CA), AESKU.NY (IFA Manufacturing and Research in Buffalo, NY).
Besides its own outstanding commitment to research and development, AESKU.GROUP initiated and supports the AESKU.KIPP INSTITUTE, an independent non-profit organization, primarily active in basic research and interdisciplinary knowledge transfer in autoimmunity.
Share article on social media or email: