What is particularly striking is that despite all these errors, since 2011, Mitkus et al. is used by CDC and other entities as the basis for claiming that aluminum adjuvants are safe.
NEWPORT BEACH, Calif. (PRWEB)
March 06, 2020
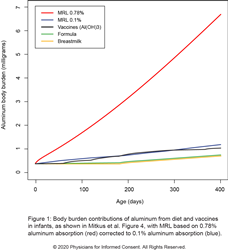
The U.S. Food and Drug Administration (FDA) and the Agency for Toxic Substances and Disease Registry (ATSDR), a division of the U.S. Department of Health and Human Services (HHS), have raised concerns about the negative effects of aluminum exposure in humans. Because some vaccines contain aluminum, the FDA published a paper in 2011 (Mitkus et al.) to address concerns about aluminum exposure from vaccines in infants. The paper compared the aluminum exposure from vaccines in infants to a safety limit of oral aluminum determined by the ATSDR. However, this study incorrectly based its calculations on 0.78% of oral aluminum being absorbed into the bloodstream rather than the value of 0.1% used by the ATSDR in its computations. As a result, the FDA paper assumed that nearly 8 (0.78%/0.1%) times more aluminum can safely enter the bloodstream, and this led the authors to incorrectly conclude that aluminum exposure from vaccines was well below the safety limit.
Physicians for Informed Consent (PIC), an educational nonprofit organization dedicated to delivering data on infectious diseases and vaccines, posted a detailed description of this error today on ResearchGate.
Dr. Christopher Shaw, professor at the University of British Columbia, has performed numerous studies on the effects of injected aluminum on mice, and commented: “We knew that the Mitkus et al. paper modeling aluminum clearance had to be inaccurate since it was assuming that injected aluminum kinetics were the same as the kinetics of aluminum acquired through diet. Now, in addition, we see that they did their modeling based on using the incorrect level of aluminum absorption. What is particularly striking is that despite all these errors, since 2011, Mitkus et al. is used by CDC and other entities as the basis for claiming that aluminum adjuvants are safe.”
Dr. Shira Miller, president of PIC, said, “We posted the Mitkus 2011 erratum on ResearchGate in hopes of bringing it to the attention of scientists and researchers who are interested in the safety of the quantities of injected aluminum found in childhood vaccines and would be in a position to further research the safety concern.”
About Physicians for Informed Consent
Physicians for Informed Consent is a 501(c)(3) educational nonprofit organization focused on science and statistics. PIC delivers data on infectious diseases and vaccines, and unites doctors, scientists, healthcare professionals, attorneys, and families who support voluntary vaccination. In addition, the PIC Coalition for Informed Consent consists of more than 200 U.S. and international organizations. To learn more or to become a member, please visit physiciansforinformedconsent.org.
Share article on social media or email: