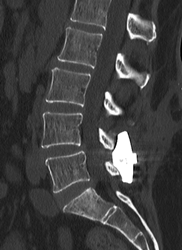
CT Scan Image of Implanted Inspan interspinous distraction decompression (IDD) Device
Dr. Raikar says, “The Inspan interspinous distraction decompression (IDD) device provides a permanent solution by stabilizing the affected spine segment for pain relief and relieving neural compression via distraction of the spinal processes and lamina from spinal stenosis.”
MALDEN, Mass. (PRWEB)
December 03, 2021
INSPAN LLC (a privately-owned company) is pleased to announce the successful results from a clinical study of outpatient interspinous lumbar fixation for degenerative intervertebral disc disorders with spinal stenosis using the Inspan interspinous fixation device (INSPAN LLC). Unlike the extension block designs, the Inspan device fixates the spine to allow immediate stability, distraction, decompression, and fusion.
The study was performed at the R Choice Surgical Center, an Outpatient Ambulatory Surgery Center in Fremont, Nebraska, USA. The contributing authors of this study are Dr. Shane V. Raikar, MD, Dr. Arun A. Patil, MD, Dr. Deepak K. Pandey, Ph.D., and Mr. Sidharta R. Kumar.
This retrospective study reports the clinical outcome of pain reduction after implanting the Inspan interspinous distraction decompression (IDD) device. The study was conducted across 13 patients with a 68-year median age, all with chronic pain, and a 19-month median follow-up time. The procedures were performed on patients diagnosed with degenerative intervertebral disc disorders and underlying spinal stenosis. There were no complications, no revisions, or any implant failures. In addition, post-op pain measured by the Numeric Pain Rating Scale (NPRS) showed statistically significant pain reduction for back and leg pain.
The lead author of the study Dr. Raikar says, “I have been managing spinal pain for my patients for last 23 years by providing them pain management solutions like Kyphoplasty, Spinal Cord Stimulator Trial and Implant, Morphine Pump Trial and Implant, Radiofrequency Nerve Ablation, and other methods that provide lasting pain relief. However, the Inspan interspinous distraction decompression (IDD) device provides a permanent solution by stabilizing the affected spine segment for pain relief and relieving neural compression via distraction of the spinal processes and lamina from spinal stenosis. The instrumentation is easy to use under fluoroscopy. The implant design allows for safe implantation to avoid spinous process fracture and subsidence.”
According to Dr. Pandey, President, and COO of Inspan LLC, “Inspan has a long clinical track record since 2010, and we have continuously incorporated feedback from our doctors to improve the device, instruments, and technique. We will continue our commitment to innovation and listen to our doctors.”
“It is very satisfying for me to train pain management physicians to use Inspan safely and effectively and to be readily available to support,” – says Prof. Dr. Kingsley R. Chin, Board Certified Orthopedic Spine Surgeon and CTO of Inspan LLC
About INSPAN, LLC
INSPAN, LLC is privately owned by the KICVentures Group and is focused on advancing the platform of patented interspinous fixation technology. The Inspan device has a proven ten-year track record with thousands implanted since FDA clearance in 2010.
https://myinspan.com/
About KICVentures Group
Our founders have been investing in spine surgery since 2000, which makes us the most experienced healthcare investment holding company with the largest portfolio of medical device technologies focused on solutions for less invasive outpatient spine surgery. Our investment strategy is to acquire or invent disruptive technologies using our own capital or partner with private individual investors. This allows us the freedom to make quick and nimble decisions such as when we acquired AxioMed Viscoelastic Disc Technologies while other companies invested in spinal fusion.
https://www.kicventuresgroup.com/
Investors
If you have any questions or comments, please contact us via email or phone, or send us a message using the contact form.
https://www.kicventuresgroup.com/contact
About R Choice Surgery Center
R Choice is a State-of-the-art outpatient Surgery Center in Fremont, NE, and Omaha, NE specializing in treatments for neuropathy, post-laminectomy syndrome, chronic neck/back pain, chronic regional pain syndrome, fibromyalgia, and headaches. R Choices’ exceptional staff provides innovative approaches and products to enhance their patient’s independence and overall function, to provide them a better quality of life.
http://www.mwa-doc.com/surgery-center/
Inspan FDA Indications
The Inspan Slim Spinous Process Plate System is a posterior non-pedicle supplemental fixation system intended for use in the non-cervical spine (T1-S1). It is intended for plate fixation/attachment to the spinous process for the purpose of achieving supplemental fusion for the following indications: spondylolisthesis, trauma (fracture or dislocation), tumor, or degenerative disc disease (defined as discogenic pain with degeneration of the disc confirmed by history and radiographic studies). The device is intended for use with bone graft material and is not intended for stand-alone use.
Publication Link – https://www.cureus.com/articles/76684-inter-spinal-fixation-and-stabilization-device-for-lumbar-radiculopathy-and-back-pain
Deepak K. Pandey, Ph.D.
Title – President & COO
Phone number: 949-632-2606
Email Address: deepakpandey@kicventures.com
Share article on social media or email: