DURHAM, N.C. (PRWEB)
March 12, 2020
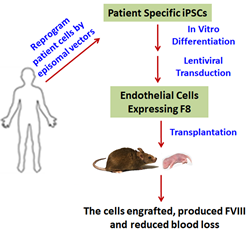
In a new study released today in STEM CELLS Translational Medicine (SCTM), researchers at the University of California, Davis and the Chinese Academy of Science demonstrate how induced pluripotent stem cells can provide a renewable supply of endothelial cells. These cells can then be genetically modified to produce high levels of a clotting protein that was used to successfully treat hemophilia A in mice.
Hemophilia A is a disorder in which the blood does not clot normally, causing people with the condition to bleed more than normal after an injury or surgery. In the most severe cases, this can lead to disability or death.
“The culprit behind hemophilia A is a protein called factor VIII (FVIII), which is responsible for blood clotting,” explained Ping Zhou, Ph.D., of UC Davis’ Stem Cell Program and co-lead investigator on the new study. “In hemophilia A patients, we find that this protein is either defective or missing altogether.”
Currently the standard treatment for hemophilia A involves repeated infusions of recombinant FVIII proteins. However, this treatment has multiple challenges. Infusion of the FVIII protein can treat, but not cure the disease. Also, the treatments must be given two to three times a week, so long-term they can be inconvenient and pose a risk of infection. In addition, this treatment is extremely costly, with the median price being $98,334 annually, according to a study published in the Journal of Medical Economics, and it is a lifelong expense. “Furthermore, bleeding episodes are still common even with factor replacement therapy due to the fluctuation of the infused FVIII levels,” said Aijun Wang, Ph.D., of the UC Davis School of Medicine and the other lead investigator on the study.
Cell therapy is actively being studied as a potential alternative therapy for hemophilia A. Multiple cell types have been tested for the delivery of FVIII in vivo with promising results. However, some major challenges remain — mainly, finding a sufficient supply of the therapeutic cells and a way to sustain their engraftment.
In the study published in SCTM, the researchers explored endothelial cells (ECs) derived from hemophilia A patients’ induced pluripotent stem cells (HA-iPSC-ECs) as a way to deliver FVIII.
“FVIII is primarily produced in ECs in a non-diseased human being, so this means that ECs hold great potential for development as a cell therapy for hemophilia A,” Dr. Wang explained. “Also, since hemophilia A is a genetic disease, a child born with the disease needs to be treated early in life. Therefore, we assessed the engraftment of the HA-iPSC-ECs at the neonatal stage in comparison to the adult stage, an analysis not previously studied. Finally, we assessed the functionality of the human HA-iPSC-ECs in attenuating hemophilia.”
To conduct their study, they collected cells from patients with hemophilia A, induced them to become induced pluripotent stem cells and then ECs and genetically modified the cells to express high levels of FVIII.
“Not only did the results produce an ample supply of ECs, but when we tested how the HA-iPSC-ECs affected mice with hemophilia A we found they were indeed capable of durable engraftment in both neonatal and adult animals. We also found that the FVIII in the cells functionally corrected or alleviated the animals’ hemophilia A symptoms,” Dr. Zhou said.
“As such, we believe that this study is a significant step forward in developing a cell therapy for hemophilia A, and that it provides proof-of concept that HA-iPSC-derived ECs can serve as an autologous cell factory to deliver FVIII for treating hemophilia A in adults and newborns, too.”
“The findings emerging from this study are interesting and useful. The opportunity of using iPSCs as a source of factor VIII producing cells that have the potential to treat hemophilia A is appealing and needs to be explored further for a potential clinical application,” said Anthony Atala, M.D., Editor-in-Chief of STEM CELLS Translational Medicine and director of the Wake Forest Institute for Regenerative Medicine.
The full article, “Endothelial Cells Derived from Patients’ Induced Pluripotent Stem Cells for Sustained FVIII Delivery and the Treatment of Hemophilia A,” can be accessed at https://stemcellsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/sctm.19-0261.
About STEM CELLS Translational Medicine: STEM CELLS Translational Medicine (SCTM), co-published by AlphaMed Press and Wiley, is a monthly peer-reviewed publication dedicated to significantly advancing the clinical utilization of stem cell molecular and cellular biology. By bridging stem cell research and clinical trials, SCTM will help move applications of these critical investigations closer to accepted best practices. SCTM is the official journal partner of Regenerative Medicine Foundation.
About AlphaMed Press: Established in 1983, AlphaMed Press with offices in Durham, NC, San Francisco, CA, and Belfast, Northern Ireland, publishes two other internationally renowned peer-reviewed journals: STEM CELLS® (http://www.StemCells.com), celebrating its 38th year, is the world’s first journal devoted to this fast paced field of research. The Oncologist® (http://www.TheOncologist.com), also a monthly peer-reviewed publication, entering its 25th year, is devoted to community and hospital-based oncologists and physicians entrusted with cancer patient care. All three journals are premier periodicals with globally recognized editorial boards dedicated to advancing knowledge and education in their focused disciplines.
About Wiley: Wiley, a global company, helps people and organizations develop the skills and knowledge they need to succeed. Our online scientific, technical, medical and scholarly journals, combined with our digital learning, assessment and certification solutions, help universities, learned societies, businesses, governments and individuals increase the academic and professional impact of their work. For more than 200 years, we have delivered consistent performance to our stakeholders. The company’s website can be accessed at http://www.wiley.com.
About Regenerative Medicine Foundation (RMF): The non-profit Regenerative Medicine Foundation fosters strategic collaborations to accelerate the development of regenerative medicine to improve health and deliver cures. RMF pursues its mission by producing its flagship World Stem Cell Summit, honouring leaders through the Stem Cell and Regenerative Medicine Action Awards, and promoting educational initiatives.