TransLoc 3D™ by CornerLoc™
TULSA, Okla. (PRWEB)
October 18, 2022
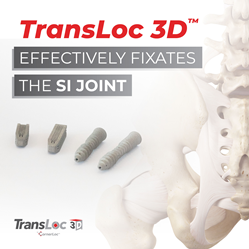
CornerLoc™ the trade name of Foundation Fusion Solutions, LLC, a medical device company, announces the much-anticipated launch of the TransLoc 3D™ SI Joint Fusion System. The TransLoc 3D™ SI Joint Fusion System is the next evolution in fixating the SI joint. The modular posterior implant and lateral oblique screw components of the TransLoc 3D™ System can be used together to create a truly 3-dimensional hybrid construct, or the screws can be used on their own as a transfixing compression construct.
“With our recent FDA 510(k) Clearance and launch of TransLoc 3D™, we are able to offer one of the most comprehensive SI joint fusion offerings available, giving the physician several implant options to match each patient’s needs. Incorporating CornerLoc’s patented and proven CornerLoc-ing technology*, together with state-of-the-art 3D printed titanium macro and micro porosity in the new ultra-porous posterior titanium implant and lateral oblique compression screw, this system offers a truly 3-dimensional approach to fusing the SI joint,” says Bob Compton, CEO and Co-founder of CornerLoc.
TransLoc 3D™ effectively fixates the SI Joint. TransLoc 3D™ Screws pass through the ilium, across the SI joint, and into the sacrum using a Lateral Oblique approach. This approach avoids sensitive anatomy, including the Gluteal artery, Gluteal nerves, and muscles that are potential obstacles of the straight Lateral approach. The TransLoc 3D™ System is intended to fixate the sacroiliac (SI) joint with the long-term goal of fusion. These titanium 3D-printed devices are available in a range of lengths and include a Posterior Implant version which can be used with the TransLoc 3D™ Screw. The TransLoc 3D™ Screws, using a Lateral Oblique approach, qualify for the CPT Panel’s new definition of transfixing**, which goes into effect January 1, 2023.
About CornerLoc™
CornerLoc™, headquartered in Tulsa, OK, was founded in 2017 on one idea: provide patients with an intelligent and lasting solution for SI joint fusion. The company has successfully been awarded a U.S. patent for the unique fixating features of theCornerLoc™ implant and has other U.S. patents pending. CornerLoc™ has offered the FDA registered CornerLoc™ graft since 2017, and has recently gained FDA 510(k) regulatory clearance for the TransLoc 3D™ system in the U.S. At CornerLoc™, we put the patient first in every decision we make and are driven by achieving positive outcomes and providing a life for our patients that is no longer dictated by chronic and debilitating pain. For more information, visit http://www.cornerloc.com.
References:
*Transfixation of the Sacroiliac Joint: Biomechanical Stability of a Dual-Implant Minimally Invasive Procedure; Antonio Valdevit, Ph.D., Steven Falowski, M.D., FAANS; SEA, Ltd., Columbus, OH, Argires-Marotti Neurosurgical Associates of Lancaster, Lancaster, PA
**New definition for transfixing to be used with Code 27279, which becomes effective January 1, 2023: placement of an internal fixation device(s) that passes through the ilium across the sacroiliac joint and into the sacrum. (CPT Category III codes long descriptors – updated July 1, 2022)
CPT © Copyright 2022 American Medical Association. All rights reserved. AMA and CPT are registered trademarks of the American Medical Association.
Share article on social media or email: