CloudLIMS helps locate COVID-19 specimens seamlessly
“As the number of COVID-19 positive cases continue to mount, accelerated clinical trials to develop a vaccine against COVID-19 are urgently needed,” said Arun Apte, Chief Executive Officer at CloudLIMS.
WILMINGTON, Del. (PRWEB)
May 10, 2020
CloudLIMS, a leading provider of laboratory informatics, announces the launch of a ready-to-go-live COVID-19 LIMS. CloudLIMS is preconfigured with CDC approved COVID-19 biorepository SOPs and can be quickly set up in less than a week.
With the global pandemic gaining a strong foothold worldwide, CloudLIMS previously announced the offering of its flagship product, CloudLIMS Enterprise, free of charge to SARS-CoV-2 & COVID-19 research, testing laboratories, and biobanks to fight the COVID-19 pandemic. CloudLIMS now invites COVID-19 biobanks to use its product to manage COVID-19 specimens and associated metadata, streamline and automate workflows right from sample accessioning, quality control tests, the shipment of specimens to researchers, and for the safe disposal of expired specimens.
Quality biobanking of COVID-19 specimens is underpinned by standardization and best practices. CloudLIMS, an in the cloud COVID-19 LIMS, enables COVID-19 biobanks to follow international standards and guidelines including ISO 20387:2018, HIPAA, EU GDPR, ISBER Best Practices to maintain quality specimens. High-quality COVID-19 specimens accelerate clinical research and trials on COVID-19, on population-based cohort studies, viral genome mutation studies, and molecular epidemiological studies.
CloudLIMS’ self-serve client portal enables researchers to place a procurement request for SARS-CoV-2 specimens and track request status in real-time. The client portal also enables researchers to place storage requests for COVID-19 specimens and attach notes for handling or storage of specimens.
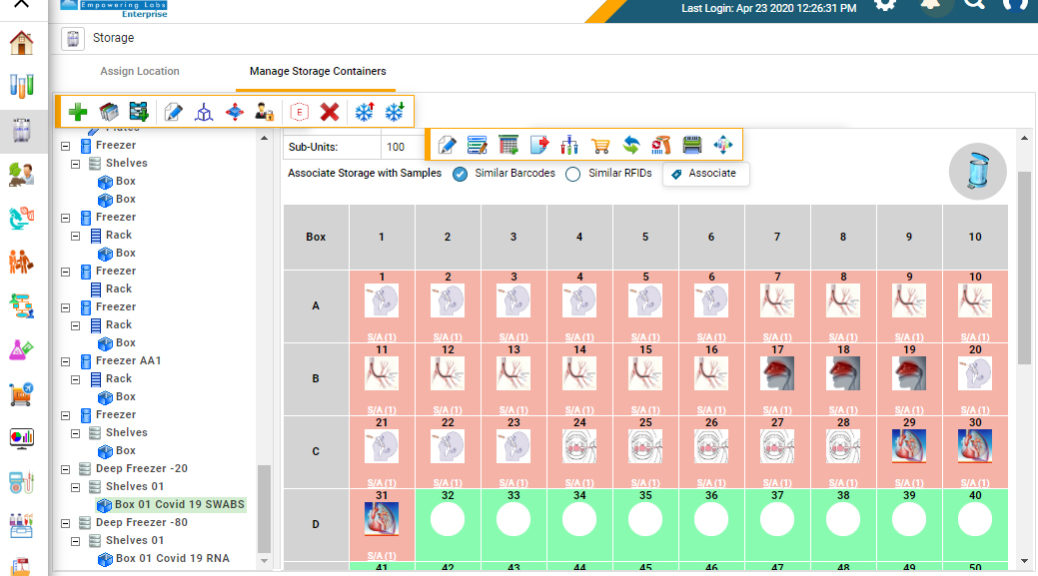
CloudLIMS enables COVID-19 biobanks to mirror their storage and inventory, automatically assign storage locations to COVID-19 specimens, enabling them to quickly locate samples and safely handle them. It also helps manage control specimens obtained from healthy individuals 2-3 months before the outbreak. COVID-19 testing laboratories use control samples to determine the reliability of test results.
COVID-19 biobanks can seamlessly manage the training and competency of their staff in handling, processing, proper packaging, and shipment of COVID-19 specimens. CloudLIMS helps biobanks manage patient consent, anonymize Protected Health Information (PHI), and generate custom reports of COVID-19 specimens, patients, and studies in a few clicks.
CloudLIMS’ Rest API is preconfigured to support integration with third-party software, such as patient registries, EMR, LIS, and instruments used in specimen quality control tests.
“As the number of COVID-19 positive cases continue to mount, accelerated clinical trials to develop a vaccine against COVID-19 are urgently needed. Biobanks serve as an indispensable tool to accelerate COVID-19 research and trials, enabling biobanks to provide needed samples for COVID-19 research. We have preconfigured CloudLIMS to support COVID-19 biobanking workflows,” said Arun Apte, Chief Executive Officer at CloudLIMS. “CloudLIMS can be deployed within a week and can help biobanks manage an unprecedented influx of COVID-19 specimens while maintaining international standards for quality biobanking,” he further added.
About CloudLIMS
Established in 2013, CloudLIMS is an energetic team of professionals producing cutting edge laboratory software solutions, such as sample management software and LIMS. For more information, please visit: http://www.cloudlims.com.
Share article on social media or email: