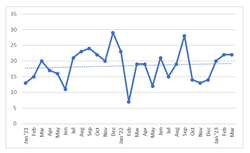
Total Number of VC/Private Funding Deals by Month, January 2021-March 2023 (total count)
“There are a lot of reasons to conclude that funding in cell and gene therapies will only strengthen in coming years,” Bruce Carlson, publisher, Kalorama Information
ARLINGTON, Va. (PRWEB)
June 20, 2023
Cell and gene therapy is attracting much attention these days and for good reason. For one, there has been a notable increase in the number of cell and gene therapies in clinical development over the past decade. In the United States alone there are over 1,000 cell and gene therapies in clinical development, and more than three times as many in pre-clinical development. But clinical and pre-clinical activity is only part of the reason to be excited about the market, considering that cell and gene therapy-related companies received more than $40 billion in funding in 2022, according to leading medical market research firm Kalorama Information.
The findings were published in Kalorama Information’s latest report Cell and Gene Therapy Market and Deals Analysis, 2023: Financings, Partnering, Mergers and Acquisitions, Tech Transfers, IPOs, and Other Deals. The report features Kalorama Information’s unique and exclusive deals tracker covering cell and gene therapy financing, collaborations, and other deals from Q1 2021-Q1 2023. The analysis is based on 1000+ deals from 2022-2023, the specific details of which are all included in the report. Deals prior to 2022 are also tabulated to present chronological trends from 2021-2023.
While the concepts of gene therapy and cell therapy have been investigated for decades, there were major challenges in the early years. Through incremental progress, and the gradual introduction of enabling tools such as CRISPR and next-generation sequencing (NGS), cell and gene therapy has emerged into a highly active area. There are now many approved therapies with proven track records. As the new tools have lowered the barriers to entry for the industry, over 1,500 companies have been created or have become involved.
Total annual cell and gene funding in 2022 dropped from around $70 billion in 2021. Partly the decrease was due to the tighter funding environment that has been seen across other industries. Some types of deals, such as collaborations and acquisitions, continue to increase significantly. The rate of new manufacturing/supply chain deals also approximately doubled. VC/private funding deals have remained at roughly the same number, although the dollar amount has dropped. The number of public offerings/SPAC deals went down in early 2022 but then grew consistently in the time since. Licensing deals have increased. Also IPOs/FPOs were initially negligible in early 2022 but have almost fully recovered in the year since. In practical terms, for cell and gene therapy companies being mostly startups, this tighter funding has been experienced as a lower average funding round for VC/private funding, while the number of those deals has remained somewhat consistent.
“Cell and gene therapy-related companies have continued to receive huge amounts of investment. And despite slight fluctuations year-to-year, there are a lot of reasons to conclude that funding in cell and gene therapies will only strengthen in coming years,” says Bruce Carlson, publisher for Kalorama Information.
Further evidence of the market’s growth momentum is linked to comments by Peter Marks of the U.S. Food and Drug Administration (FDA), who in March 2023 expressed a desire to boost commercialization for cell and gene therapies for patients with rare diseases by aligning global regulations.
At the Reuters Pharma USA conference in March in Philadelphia, Marks commented: “If you could have a way of having the EU, U.S., Japan, Canada, perhaps Switzerland all have approvals at close to the same time, that would give a market that would be four or five times as large as just the U.S.”
For more information or to purchase the market research report “Cell and Gene Therapy Market and Deals Analysis, 2023: Financings, Partnering, Mergers and Acquisitions, Tech Transfers, IPOs, and Other Deals”, visit https://kaloramainformation.com/product/cell-and-gene-therapy-market-and-deals-analysis-2023-financings-partnering-mergers-and-acquisitions-tech-transfers-ipos-and-other-deals/.
ABOUT KALORAMA INFORMATION
Kalorama Information, part of Science and Medicine Group, is the leading publisher of market research in healthcare areas, including in vitro diagnostics (IVD), biotechnology, medical devices, and pharmaceuticals. Science and Medicine Group supports companies seeking to commercialize the rapidly changing marketplace at the intersection of science, medicine, and technology. Comprised of industry-leading brands, Science and Medicine Group serves analytical instrument, life science, imaging, and clinical diagnostic companies by helping them create strategies and products to win markets and provide platforms to digitally engage their markets through a variety of innovative solutions. Kalorama Information produces 30 reports a year. The firm offers a Knowledge Center, which provides access to all published reports.
Share article on social media or email: