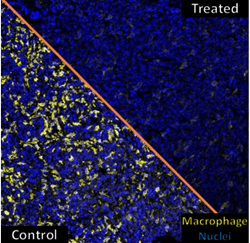
Mice with lung cancer were treated with macrophage-targeting CAR T cells resulting in reduced numbers of tumor macrophages (right side), shrunken tumors, and extended survival.
“Our initial goal was just to use the CAR T cells to kill the immunosuppressive macrophages, but we discovered they were also boosting tumor immunity by releasing this powerful immune-boosting molecule,” – Brian Brown, PhD
NEW YORK (PRWEB)
October 25, 2022
A new approach to cancer immunotherapy that uses one type of immune cell to kill another—rather than directly attacking the cancer—provokes a robust anti-tumor immune response that shrinks ovarian, lung, and pancreatic tumors in preclinical disease models, according to researchers at the Icahn School of Medicine at Mount Sinai in New York. The findings were published October 11, 2022 in the journal Cancer Immunology Research [https://doi.org/10.1158/2326-6066.CIR-21-1075 .
The study involved a twist on a type of therapy that uses immune cells known as CAR T cells. CAR T cells in current clinical use are engineered to recognize cancer cells directly and have successfully treated several blood cancers. But there have been challenges that prevent their effective use in many solid tumors.
Most solid tumors are heavily infiltrated by a different type of immune cell called macrophages. Macrophages help tumors grow by blocking the entry of T cells into tumor tissue, which prevents CAR T cells and the patient’s own T cells from destroying the cancer cells.
To tackle this immune suppression at the source, the researchers engineered T cells to make a “chimeric antigen receptor” (CAR) that recognizes a molecule on the surface of macrophages. When these CAR T cells encountered a tumor macrophage, the CAR T cell became activated and killed the tumor macrophage.
Treatment of mice bearing ovarian, lung, and pancreatic tumors with these macrophage-targeting CAR T cells reduced the number of tumor macrophages, shrunk the tumors, and extended their survival.
The killing of tumor macrophages allowed the mouse’s own T cells to access and kill the cancer cells. The investigators further demonstrated that this anti-tumor immunity was driven by release of the cytokine interferon-gamma—a molecule involved in the regulation of inflammatory responses—from the CAR T cells.
“Our initial goal was just to use the CAR T cells to kill the immunosuppressive macrophages, but we discovered they were also boosting tumor immunity by releasing this powerful immune-boosting molecule,” said senior author Brian Brown, PhD, Director of the Icahn Genomics Institute and Associate Director of the Marc and Jennifer Lipschultz Precision Immunology Institute (PrIISM) at Icahn Mount Sinai. “It was a one-two punch from this single treatment.”
Shifting the sights of CARs from cancer cells to tumor macrophages potentially addresses another key barrier to the successful elimination of solid tumors with CAR T cells. There are very few proteins found exclusively on cancer cells and not on healthy tissues that can be used to target cancer cells in solid tumors directly without damaging the healthy tissue.
The macrophages found in tumors that suppress immunity are very similar across different types of cancer and very different from macrophages in healthy tissues.
This has led to an interest in macrophage-depleting agents for cancer therapy, but approaches developed to date have had limited success in clinical trials.
“Our molecular studies of human tumors have revealed macrophage subsets present in human tumors and not in normal tissues and are similar across tumors and across patients. So macrophage-targeting CAR T cells could be a broad way to target different types of solid tumors and improve immunotherapy,” said Miriam Merad, MD, PhD, co-senior author of the study, and Director of PrIISM.
Next, the researchers are working on tumor macrophage-specific CAR and generating humanized versions of the genetic instructions, so that they can be introduced into cancer patients’ own T cells.
The paper is titled, “Targeting macrophages with CAR T cells delays solid tumor progression and enhances anti-tumor immunity.”
Additional co-authors are Alfonso R. Sánchez-Paulete, PhD, Jaime Mateus-Tique, Gurkan Mollaoglu, PhD, , Sebastian R. Nielsen, PhD, Adam Marks, Ashwitha Lakshmi, Jalal A. Khan, MD, PhD, C. Matthias Wilk, MD, Luisanna Pia, Alessia Baccarini, PhD, all from Icahn Mount Sinai.
B.D.B. was supported by NIH (R01CA257195) and a grant from the Alliance for Cancer Gene Therapy. M.M. was supported by NIH (R01CA254104). A grant from the Applebaum Foundation also supported the project. A.R.S.P. was supported by a grant from Fundación Alfonso Martín Escudero (Spain).
About the Icahn School of Medicine at Mount Sinai
The Icahn School of Medicine at Mount Sinai is internationally renowned for its outstanding research, educational, and clinical care programs. It is the sole academic partner for the eight- member hospitals* of the Mount Sinai Health System, one of the largest academic health systems in the United States, providing care to a large and diverse patient population.
Ranked 14th nationwide in National Institutes of Health (NIH) funding and among the 99th percentile in research dollars per investigator according to the Association of American Medical Colleges, Icahn Mount Sinai has a talented, productive, and successful faculty. More than 3,000 full-time scientists, educators, and clinicians work within and across 34 academic departments and 35 multidisciplinary institutes, a structure that facilitates tremendous collaboration and synergy. Our emphasis on translational research and therapeutics is evident in such diverse areas as genomics/big data, virology, neuroscience, cardiology, geriatrics, as well as gastrointestinal and liver diseases.
Icahn Mount Sinai offers highly competitive MD, PhD, and Master’s degree programs, with current enrollment of approximately 1,300 students. It has the largest graduate medical education program in the country, with more than 2,000 clinical residents and fellows training throughout the Health System. In addition, more than 550 postdoctoral research fellows are in training within the Health System.
A culture of innovation and discovery permeates every Icahn Mount Sinai program. Mount Sinai’s technology transfer office, one of the largest in the country, partners with faculty and trainees to pursue optimal commercialization of intellectual property to ensure that Mount Sinai discoveries and innovations translate into healthcare products and services that benefit the public.
Icahn Mount Sinai’s commitment to breakthrough science and clinical care is enhanced by academic affiliations that supplement and complement the School’s programs.
Through the Mount Sinai Innovation Partners (MSIP), the Health System facilitates the real-world application and commercialization of medical breakthroughs made at Mount Sinai. Additionally, MSIP develops research partnerships with industry leaders such as Merck & Co., AstraZeneca, Novo Nordisk, and others.
The Icahn School of Medicine at Mount Sinai is located in New York City on the border between the Upper East Side and East Harlem, and classroom teaching takes place on a campus facing Central Park. Icahn Mount Sinai’s location offers many opportunities to interact with and care for diverse communities. Learning extends well beyond the borders of our physical campus, to the eight hospitals of the Mount Sinai Health System, our academic affiliates, and globally.
Mount Sinai Health System member hospitals: The Mount Sinai Hospital; Mount Sinai Beth Israel; Mount Sinai Brooklyn; Mount Sinai Morningside; Mount Sinai Queens; Mount Sinai South Nassau; Mount Sinai West; and New York Eye and Ear Infirmary of Mount Sinai.