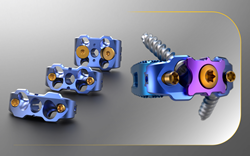
The MONDRIAN™ ALIF Cage System with Supplementary Fixation Plate features TiCro™ surface technology, which significantly increases endplate contact surface area and enhances interlocking properties.
This innovative product is designed to provide exceptional anterior column stabilization and supplemental fixation, while providing operative flexibility and accommodating a wider range of patient anatomies.
DALLAS (PRWEB)
October 11, 2022
CTL Amedica Corporation has received official 510k clearance from the U.S. Food and Drug Administration (FDA) to market its MONDRIAN™ Anterior Lumbar Interbody Fusion (ALIF) Cage System with Supplementary Fixation Plate. The MONDRIAN™ ALIF Cage System with Supplementary Fixation Plate, designated K213641/S003, is an integrated plate-and-cage construct, that can be manufactured in PEEK, titanium and a hybrid titanium-plated PEEK. The system, a standalone extension to the already robust MONDRIAN™ offering, features CTL Amedica’s proprietary TiCro™ surface technology, which significantly increases endplate contact surface area and enhances interlocking properties.
“This innovative product is designed to provide exceptional anterior column stabilization and supplemental fixation, while providing operative flexibility and accommodating a wider range of patient anatomies,” said Daniel Chon, CEO of CTL Amedica. “We’re excited about the FDA approval and look forward to introducing the MONDRIAN™ ALIF Cage System with Supplementary Fixation Plate to the industry this week.”
The MONDRIAN™ ALIF Cage System with Supplementary Fixation Plate features an efficient screw-locking mechanism, a supplementary screw-blocking plate for additional reinforcement, a significant lateral aperture for improved imaging, and a large central chamber that maximizes graft volume. The system introduces hyperlordotic offerings to the CTL Amedica portfolio and features a smooth and tapered leading-edge for easy insertion and multiple screw plate configurations to better accommodate patient anatomy and surgical preference.
CTL Amedica is a forward-thinking medical device design, development and manufacturing company. CTL Amedica maintains a Texas-based headquarters and in-house manufacturing facility, along with a Pennsylvania-based R&D Center of Excellence. A leader in the medical device technology and biomaterials space, CTL Amedica provides a full line of cervical, thoracic and lumbar fusion and fixation products. In addition, it is the world’s exclusive provider of silicon nitride spine products. Silicon nitride demonstrates an enhanced osteogenic response for enhanced fusion, promotes unique anti-bacterial properties and provides comprehensive imaging across all modalities. For more information, visit https://www.ctlamedica.com/.
Share article on social media or email: