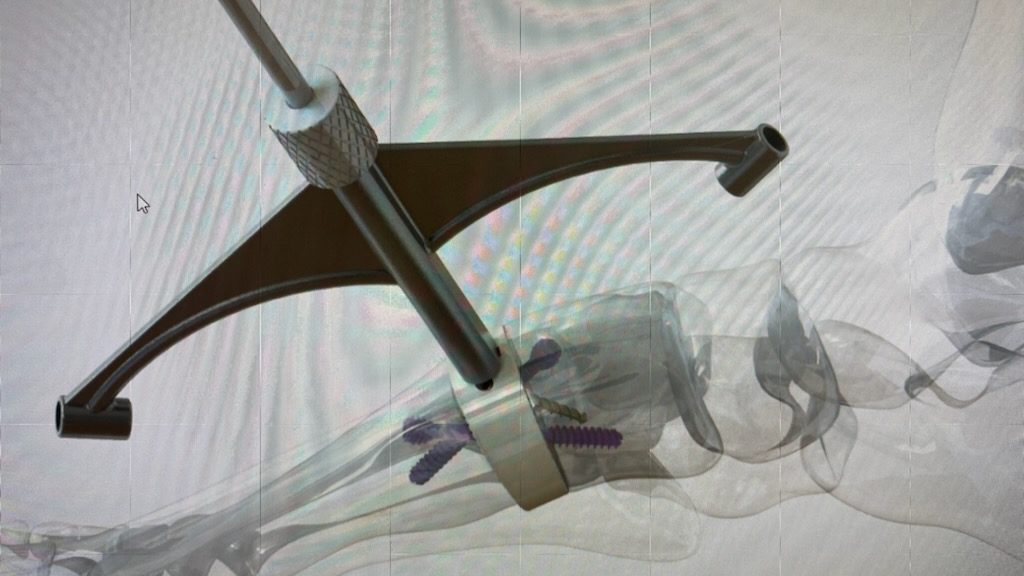
Trigon Lapidus Wedge
The Trigon Lapidus Wedge allows for reproducible triplanar correction, length restoration, and anatomical realignment.
SAN ANTONIO (PRWEB)
February 25, 2022
Nvision Biomedical Technologies has received FDA Clearance for the first use of PEEK-OPTIMA® HA Enhanced in Lapidus and Subtalar Fusion Wedges. Through the expansion of its Trigon® product line, these wedges are the fifth and sixth medical devices cleared by Nvision utilizing PEEK-OPTIMA® HA Enhanced. This polymer from Invibio Biomaterial Solutions promotes multi-directional bone healing and allows for improved fixation. Additionally, the Trigon Lapidus Wedge is the first implant to specifically reference lengthening in its FDA indication.
“We are always looking at new ways to address different foot and ankle procedures that will benefit the patient and surgeon,” said Nvision’s Senior Vice President of Product Development, Tom Zink. “By taking an innovative approach to marrying the best materials, manufacturing platforms, and engineering science, we are changing the conversation around foot and ankle surgery.”
The Trigon Lapidus Wedge is a PEEK-OPTIMA HA Enhanced implant indicated for a first metatarsal-cuneiform lengthening arthrodesis. It is offered in three footprint sizes with various length restoring thicknesses, as well as variations in sagittal and transverse angle correction. Additionally, the system provides a jig that allows for frontal plane rotation, resulting in triplanar correction with the ability to restore or maintain length of the first metatarsal.
“Most bunion osteotomies address the cosmetic bump at the first MPJ by cutting and shifting the head of the first metatarsal in 2D. However, the majority of bunions have a frontal plane 3D rotational deformity,” said Walter W. Strash, D.P.M., FACFAS. “The Nvision Trigon Lapidus Wedge enables the foot and ankle surgeon to address the bunion deformity at the TMT joint where it occurs. The Trigon Lapidus Wedge allows for reproducible triplanar correction, length restoration, and anatomical realignment.”
The Trigon Stand-Alone Subtalar Wedge is the first PEEK-OPTIMA HA Enhanced wedge for Subtalar Fusion in the market. The PEEK-OPTIMA HA Enhanced material, which has a modulus similar to bone, allows artifact-free imaging and provides an osteoconductive surface for bone ongrowth. With a 25mm diameter, the Subtalar Wedge offers correction heights ranging from 6mm to 16mm in parallel and angled options.
“The Trigon Subtalar Wedge provides the ability to rotate the wedge after insertion allowing the surgeon to optimally position the calcaneous relative to the talus,” said Mica Murdoch D.P.M., FACFAS. “In addition, the properties of PEEK-OPTIMA HA Enhanced result in faster fusion times and provide better bone apposition than a metal implant, increasing the chance of a successful correction.”
Nvision, founded in 2013, is quickly becoming the largest medical device manufacturer in San Antonio, with 23 FDA-cleared devices across a range of orthopedic specialties. In the last four months, Nvision added four products with FDA clearance to its portfolio, with one currently in FDA review and two more scheduled for submission. Nvision has 18 issued patents with more than 30 in development.
About Nvision Biomedical Technologies
Nvision is a San Antonio-based medical device and implant manufacturer focused on providing surgeons with implants paired with instrumentation and biologics that simplify and improve surgical procedures to help patients get back their quality of life. Nvision is committed to developing and manufacturing thoughtfully designed products combined with exceptional service that keep patients, surgeons, healthcare providers, and distribution partners in mind.
Nvision is aligned with surgeon thought leaders, key researchers, and development engineers from prestigious institutions to design, test, and bring to market the intuitive, intelligent, and integrated concepts. Find out more at http://www.nvisionbiomed.com
Share article on social media or email: