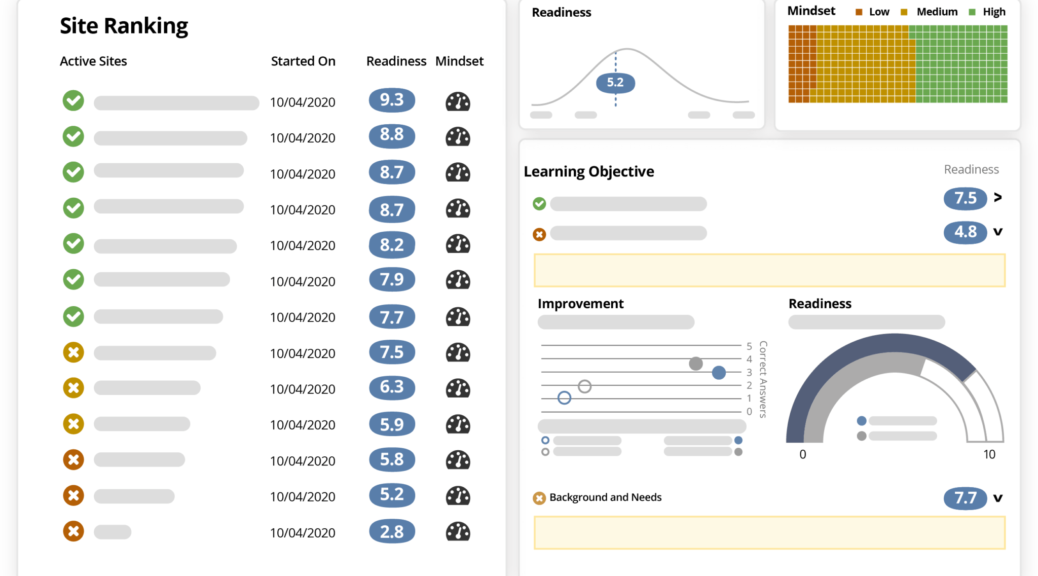
ValenzaBio is deploying Ready to transform the critical phases of study start-up and on-going site engagement for their VB119 study
CHARLOTTESVILLE, Va. (PRWEB)
April 21, 2021
ArcheMedX announced today that ValenzaBio, a biotech focused on developing monoclonal antibody therapeutics for autoimmune and inflammatory indications, has selected Ready, the industry’s leading clinical trial learning and predictive analytics platform, to optimize performance on its upcoming Phase II study.
ValenzaBio is deploying Ready to transform the critical phases of study start-up and on-going site engagement for their VB119 study. With Ready, ValenzaBio will avoid the typical delays that impede 85% of all clinical trials by implementing an on-demand, clinical trial readiness solution to increase the preparedness of its trial team and study sites.
“The ValenzaBio team is aggressively moving our research forward for monoclonal antibodies. To continue this pace during these uncertain times, we knew we wanted a more innovative and future-proof approach to the next phase studies,” said Jay Mitchell, VP, Clinical Operations at ValenzaBio. “We selected the Ready platform because we could see the immediate and long-term benefits in performance and efficiency, cost reduction, and improved oversight into our CRO relationship.”
Ready by ArcheMedX enables life sciences organizations to better equip and evaluate trial teams and sites, revealing those most prepared to start and effectively conduct the study. By transforming study documents into interactive learning experiences, Ready helps to eliminate manual tasks as it virtualizes start-up activities and automates upskilling over time. The predictive insights Ready provides allows CROs and trial sponsors to prioritize site activation, accelerate enrollment, and avoid preventable delays throughout the study.
“We are thrilled to add ValenzaBio to our growing list of forward-thinking pharma, biotech, medical device, and CRO clients,” said Joel Selzer, CEO of ArcheMedX. “Trial sponsors and CROs must confront the dual challenge of competing in an already difficult market and overcoming operational barriers still posed by the pandemic. We enable our clients to adapt and innovate, and ValenzaBio is already leaping ahead.”
As ValenzaBio deploys Ready, the biotech will:
- Eliminate most or all of the traditional travel- and hosting-related costs associated with primary investigator meetings and site initiation visits.
- Significantly reduce costs related to turnover, initiating sites or CRO resources that are not ready, and performance-related delays.
- Gain visibility to both CRO and site staff preparedness, areas of risk, and content deficiency.
- Reduce site burden by enabling an on-demand asynchronous solution to deliver investigator training and preparing site members.
- Improve site engagement by increasing retention of study knowledge, and improving understanding of study changes.
- Better inform operational decisions through predictive insights.
About ArcheMedX:
ArcheMedX helps companies across the life sciences and healthcare industries to better equip, evaluate, and predict team and clinician performance, in order to accelerate the development and adoption of new clinical treatments and best practices.
Ready by ArcheMedX is an industry leading solution that predicts and improves clinical trial performance. The platform applies behavioral science to enhance how trial team members and site personnel will apply knowledge and skills in real-world scenarios. Ready then analyzes unique behavioral indicators to reveal areas of trial readiness and potential risk.
To learn more about our readiness solutions across clinical operations, commercial programs, and medical education, visit http://www.archemedx.com or follow ArcheMedX on LinkedIn.
About ValenzaBio:
ValenzaBio is a privately held biopharmaceutical company focused on developing safe and effective therapies for autoimmune and inflammatory diseases. The company is advancing a pipeline of differentiated monoclonal antibodies targeting clinically validated mechanisms of action, in order to provide improved therapies for patients with limited treatment options. ValenzaBio’s lead program, VB119, is being developed for the treatment of membranous nephropathy (MN), followed by VB421, which is being developed for the treatment of thyroid eye disease. ValenzaBio is based in Bethesda, Md. For more information, please visit http://www.valenzabiotech.com.
Share article on social media or email: